All RSC Education articles in Non-EiC content – Page 46
-
 Resource
ResourceBasic mathematical competencies starters | 16–18
Use these Starters for ten to gauge your student’s grasp of basic mathematical competencies including rearranging equations, significant figures and unit conversions.
-
 Resource
ResourceBasic chemistry competencies starters | 16–18
Test your students’ abilities to balance equations, construct ionic formulae and write equations from text using our basic chemistry competencies Starters for ten questions.
-
 Resource
ResourceChromatography: Techniques
Learn about two fundamental techniques underpinned by chromatography: high performance liquid chromatography (HPLC) and gas chromatography–mass spectrometry (GC-MS)
-
 Resource
ResourceNuclear magnetic resonance (NMR) spectroscopy: Hydrogen
Numclear magnetic resonance (NMR) is particularly useful in the identification of the positions of hydrogen atoms (1H) in molecules. This is an invaluable technique in the identification of organic compounds and commonly used in analytical laboratories
-
 Resource
ResourceInternational Year of the Periodic Table activities
In celebration of the International Year of the Periodic Table, explore the chemical elements using innovative and interactive resources
-
 Resource
ResourceIYPT activities: periodic table bingo
An exciting take on an important topic – periodic table bingo is a fun and engaging way to learn about the elements, their symbols and their uses. It can be differentiated to varying abilities and adapted to different levels or specifications
-
 Resource
ResourceIYPT activities: build an atom simulator activity sheet
Visualise the abstract topic of atomic structure with an interactive simulation. The activity sheet gives a structured plan which can be used to focus independent exploration
-
 Resource
ResourceUltraviolet–visible (UV-vis) spectroscopy: Explanation of colour
Why do some compounds appear certain colours? The electron configuration of transition metal complexes is essential in understanding their behaviour. Understand the theory of how d-orbitals influence colour through their shape and crystal field splitting
-
 Resource
ResourceUltraviolet–visible spectroscopy (UV-vis): The origin of colour in organic compounds
DIscover how unsaturation in organic compounds leads to colour. Such electon configuration allows transitions between orbitals of lower energy and antibonding orbitals occur when electromagnetic radiation of suitable energy is absorbed by the molecule.
-
 Resource
ResourceUltraviolet–visible (UV-vis) spectroscopy: Colour in transition metal compounds
Transition elements are found in the d-block of the periodic table and the most interesting feature of transition metal compounds is that most are highly coloured.
-
 Resource
ResourceInfrared (IR) spectroscopy: More complicated molecules
Learn about the fundamental physics responsible for the IR spectra of more complicated molecules. Bringing together vibrational modes, bond strengths and dipole moments — and how these translate to the recorded spectra.
-
 Resource
ResourceInfrared (IR) spectroscopy: Energy levels
Infrared spectroscopy reflects the type of bonding present within a molecule, learn how the energy levels of bond vibrations and dipole moments contribute to the frequencies observed
-
 Resource
ResourceInfrared (IR) spectroscopy: Uses of IR spectroscopy
Infrared spectroscopy is a valuable technique in analytical chemistry. Learn about how spectra arise and the instruments used to measure them
-
 Resource
ResourceInfrared (IR) spectroscopy
Absorption of infrared radiation brings about changes in molecular vibrations within molecules and ‘measurements’ of the ways in which bonds vibrate gives rise to infrared spectroscopy. Atom size, bond length and bond strength vary in molecules and so the frequency at which a particular bond absorbs infrared radiation will be different over a range of bonds and modes of vibration.
-
 Resource
ResourceIntroduction to spectroscopy
Get back to basics with this primer on the principles of spectroscopic techniques, including infrared (IR), ultraviolet-visible (UV-vis) and nuclear magnetic resonance (NMR). To make it even easier, each technique has clear explanations and descriptions supported by animations.
-
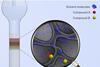 Resource
ResourceChromatography
Chromatography covers a broad range of physical methods used to separate and/or analyse complex mixtures. It can be preparative or analytical and has a wide range of applications.
-
 Resource
ResourceUltraviolet–visible (UV-vis) spectroscopy
Learn how UV-visible radiation can be used to shed light on chemical identification and how our senses percept colour. From the theory behind molecular orbitals and electronic transitions to the application of this technique with relatable examples. Includes examples and interactive simulations to aid understanding.
-
 Resource
ResourceMass spectrometry (MS)
Mass spectrometry is a powerful technique in the modern analytic laboratory. Learn the fundamental theory behind the operation of a mass spectrometer.
-
 Resource
ResourceNuclear magnetic resonance (NMR) spectroscopy
Discover how nuclear magnetic resonance (NMR) spectroscopy works, with this series of topics breaking down the fundamental theory. Covering the electronic environment of atoms right up to demonstrating the practical identification of molecules. Includes examples and interactive simulations to aid understanding.
-
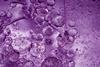 Class experiment
Class experimentElectrolysis of brine
Use this colourful practical to introduce students to the electrolysis of brine, or sodium chloride solution. Includes kit list and safety instructions.











